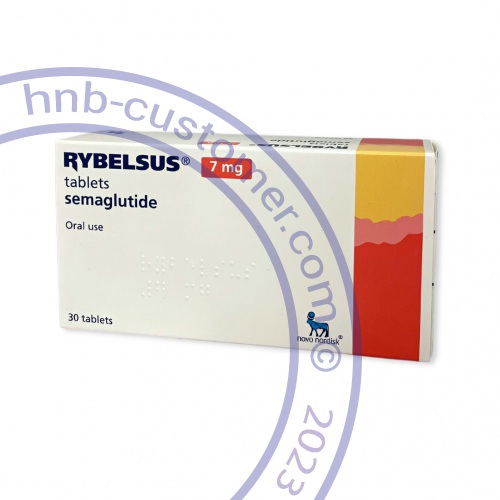
Rybelsus®
Semaglutide
Rybelsus® tablets
What is Rybelsus?
Rybelsus tablets (semaglutide) is a prescription medicine used in adults with type 2 diabetes mellitus to decrease blood sugar levels. Rybelsus can be prescribed when other diabetes medicines have been tried without success, and should be used together with diet and exercise.
Rybelsus tablets work to lower blood sugar (glucose) levels by increasing how much insulin is released and decreasing glucagon secretion. This medicine also may slow gastric emptying after eating. Rybelsus is from the class of medicines called GLP-1 receptor agonists.
Rybelsus is not for treating type 1 diabetes.
Rybelsus was first approved by the FDA on September 20, 2019.
Warnings
You should not use Rybelsus if you have multiple endocrine neoplasia type 2 (tumors in your glands), a personal or family history of medullary thyroid cancer, insulin-dependent diabetes, or diabetic ketoacidosis.
Call your doctor at once if you have signs of a thyroid tumor, such as swelling or a lump in your neck, trouble swallowing, a hoarse voice, or shortness of breath.